
Taken together, this is potentially ground breaking and practice-changing work.

Keynote 407 trial#
The KEYNOTE-189 trial looked at traditional combination chemotherapy versus this combination plus pembrolizumab and found significant improvement in several reported outcomes with the addition of pembrolizumab. The KEYNOTE-042 trial found that pembrolizumab as a first-line treatment of locally advanced or metastatic disease resulted in significantly longer overall survival compared with platinum-based chemotherapy. This article reviews several recently presented studies utilizing pembrolizumab, which is a PD-1 inhibitor. doi:10.1200/JCO.2018.36.15_suppl.Immune checkpoint inhibition by monoclonal antibodies is a quickly advancing area of non-small cell lung cancer (NSCLC) research and treatment. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. Then are hypophysitis, and nephritis occurring in 0.7% of patients.ġ.
Keynote 407 skin#
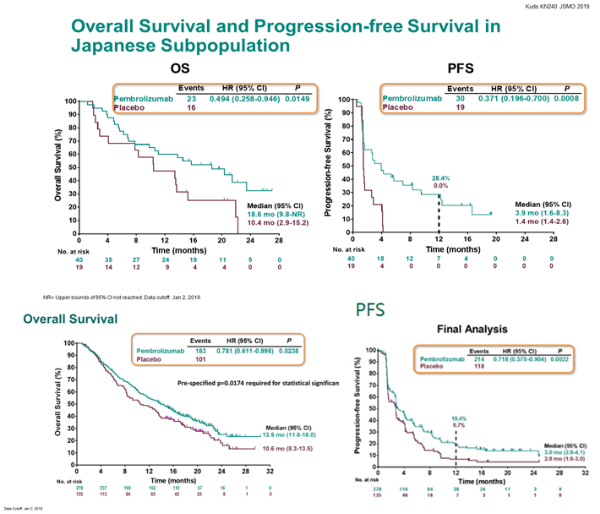
2 There was also some hyperthyroidism at, pneumonitis, colitis occurring in followed by hepatitis in, and skin reactions. Hypothyroidism was the most common immune-mediated AE, in the pembrolizumab plus chemotherapy group. The all-cause adverse events occurring in at least 20% of patients as well as immune-mediated AEs and infusion reactions. How did the toxicity of the KEYNOTE-407 regimen affect patients? Then for patients who had greater than 50% PD-L1 expression, it’s 8.0 months for pembrolizumab versus 4.2 months for chemotherapy. The median PFS was 7.2 months for those who received pembrolizumab versus 5.2 for chemotherapy. PFS by PD-L1 expression subgroup, PD-L1–negative patients had superior outcomes with pembrolizumab plus chemotherapy compared with chemotherapy alone, with a median PFS of 6.3 months for pembrolizumab versus 5.3 motnhs with chemotherapy for patients with PD-L1 expression of 1% to 49%. Median OS was not reached for the PD-L1–high group. For the PD-L1–low group, median OS is 14.0 months with pembrolizumab versus 11.6 months. We saw something similar for PD-L1–low versus PD-L1–negative, where we’re seeing a better survival among the patients who have negative PD-L1 expression. The median OS for patients with was 15.9 months versus months for patients who didn’t receive pembrolizumab. One caveat to mention is that the number of patients with a PD-L1 score of 50% or greater was lower than the other 2 groups, and so the confidence interval crossed 1.00 you have to take into account the number of patients in that group.īut otherwise, for patients with PD-L1–negative expression or positive expression of 1% to 49%, pembrolizumab plus chemotherapy was significantly associated with better OS. Again, there was a separation of the Kaplan-Meier curve favoring pembrolizumab plus chemotherapy in all groups. tumor PD-L1 expression subgroups in PD-L1–negative, PD-L1–low, and PD-L1–high patients. For pretty much all of the groups, the pembrolizumab combination was favored for OS. OS subgroup analyses took into account age, sex, performance status, region of enrollment, tumor proportion score/PD-L1 score, as well as the 2 different taxane-based regimens. What was reported about the subgroup analyses of the primary end points, especially PD-L1 score? 1 The median PFS for pembrolizumab plus chemotherapy was 6.4 months versus 4.8 months for chemotherapy alone, with a hazard ratio of 0.56 significantly in favor of pembrolizumab plus chemotherapy as well. The OS had a hazard ratio of 0.64 in favor of the pembrolizumab plus chemotherapy group, and a median OS of 15.9 months versus 11.3 months for patients who received chemotherapy alone.
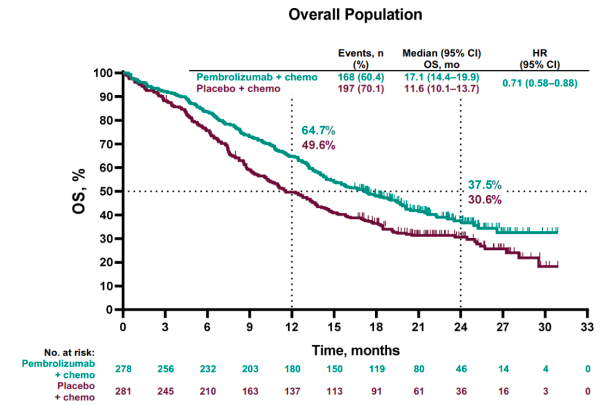
What were the findings for OS and PFS in this study?ĭata were presented from the second interim analysis on OS and PFS.

The primary end points were progression-free survival and overall survival, and the secondary end points were objective response rate, duration of response, and safety. This study randomly assigned patients to 200 mg of pembrolizumab every 3 weeks with carboplatin and paclitaxel or nab-paclitaxel for 4 cycles versus only carboplatin and paclitaxel or nab-paclitaxel for 4 cycles. SCHOENFELD: This is a first-line chemotherapy with or without pembrolizumab in stage IV squamous NSCLC. Targeted Oncology TM: Please describe the study design and goals of the KEYNOTE-407 trial (NCT02775435) for patients with advanced squamous cell non–small cell lung cancer (NSCLC).


 0 kommentar(er)
0 kommentar(er)
